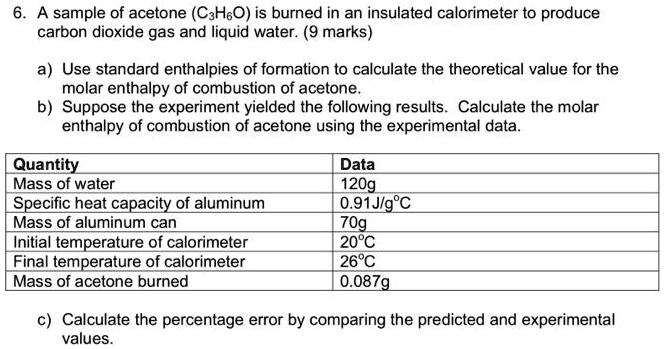
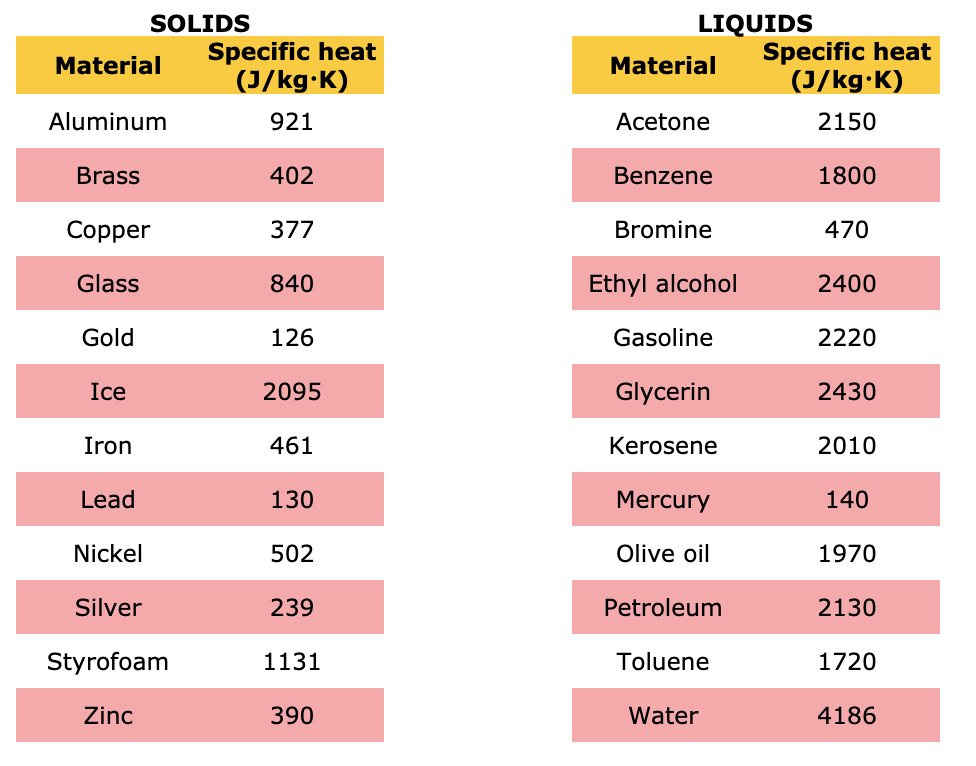
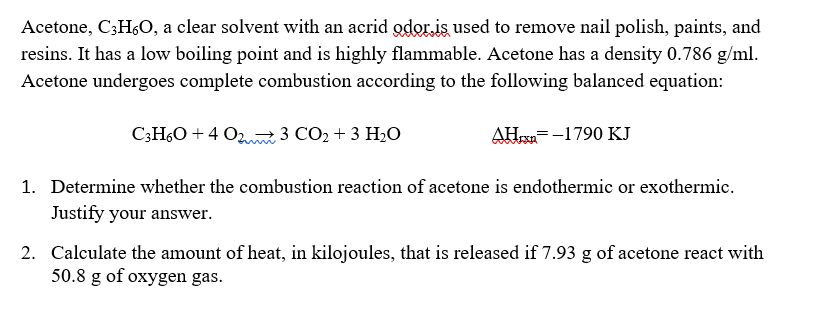
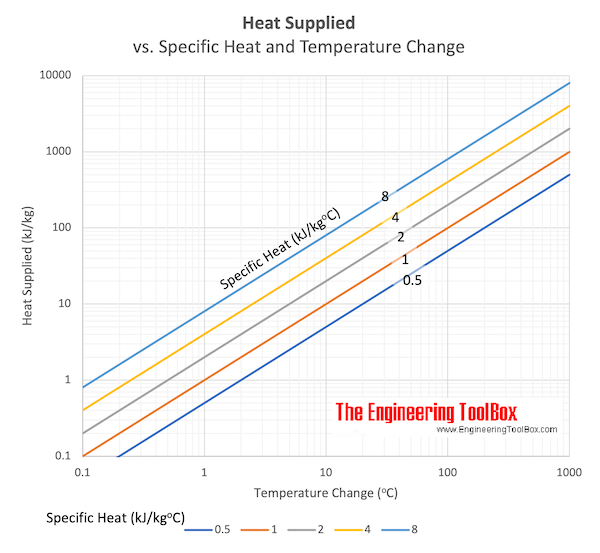
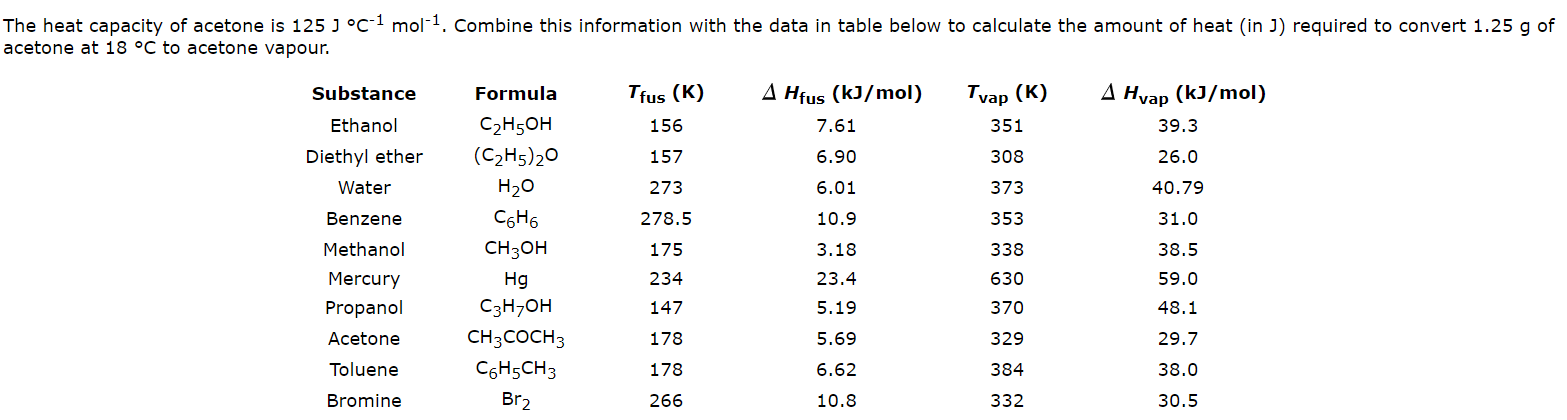
Using the given data and calculate the enthalpy of formation of acetone(g). Bond enthalpy of : C - H = 415 ; C - C = 350 ; (C = O) =
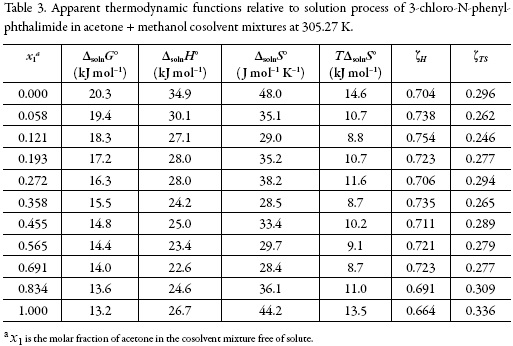
Solution thermodynamics and preferential solvation of 3-chloro-N-phenyl-phthalimide in acetone + methanol mixtures
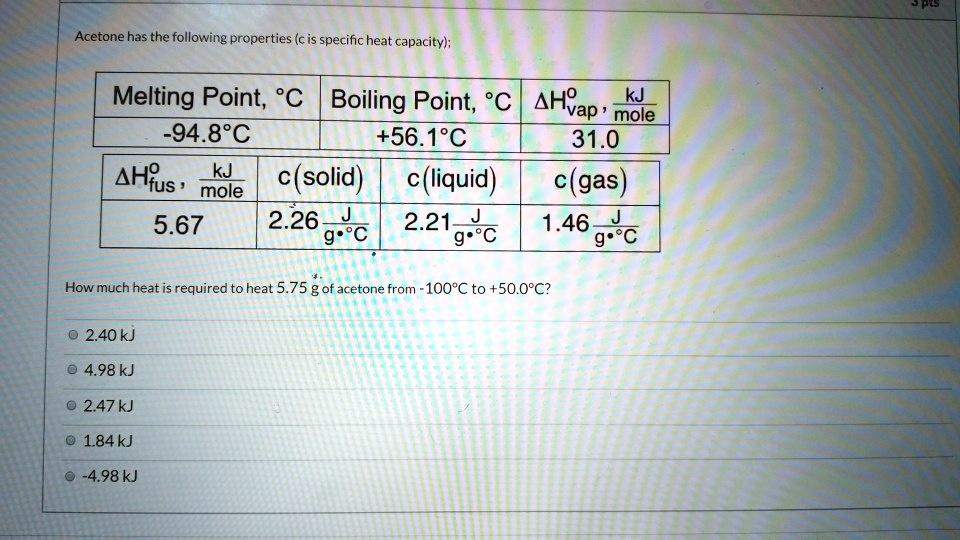
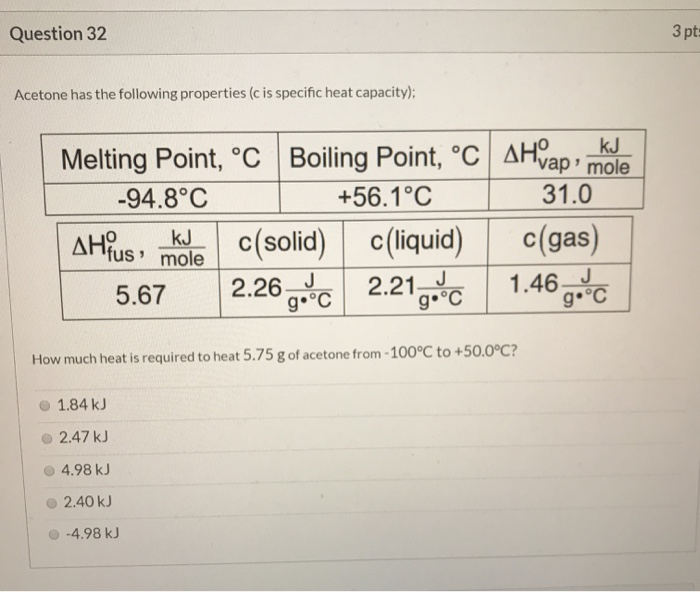
SOLVED: Acetone has the following properties (c is specific heat capacity); Melting Point; %C Boiling Point; C AHYap' kJ mole -94.8'C +56.1*C 31.0 AH?s , kJ mole (solid) (liquid) c(gas) 5.67 2.26-
THE HEAT CAPACITIES OF ISOPROPYL ALCOHOL AND ACETONE FROM 16 TO 298°K. AND THE CORRESPONDING ENTROPIES AND FREE ENERGIES | Journal of the American Chemical Society

Acetone heat capacities: (c L and c G ) for the molecular liquid and... | Download Scientific Diagram

Heat of vaporization of acetone. Simulation data: • this work, AUA4... | Download Scientific Diagram

SOLVED: A solid substance has a mass 0f 250.00g It is cooled by 25.00*C and loses 4.937kJ of heat What is the specific heat capacity of the substance in 9.PC [APP 2